NORTH CAROLINA -- Wake Research has announced the start of several new clinical research trials that will help battle the coronavirus pandemic.
The trials will include vaccines for COVID-19, coronavirus diagnostic testing, and inpatient and outpatient care for patients with the virus.
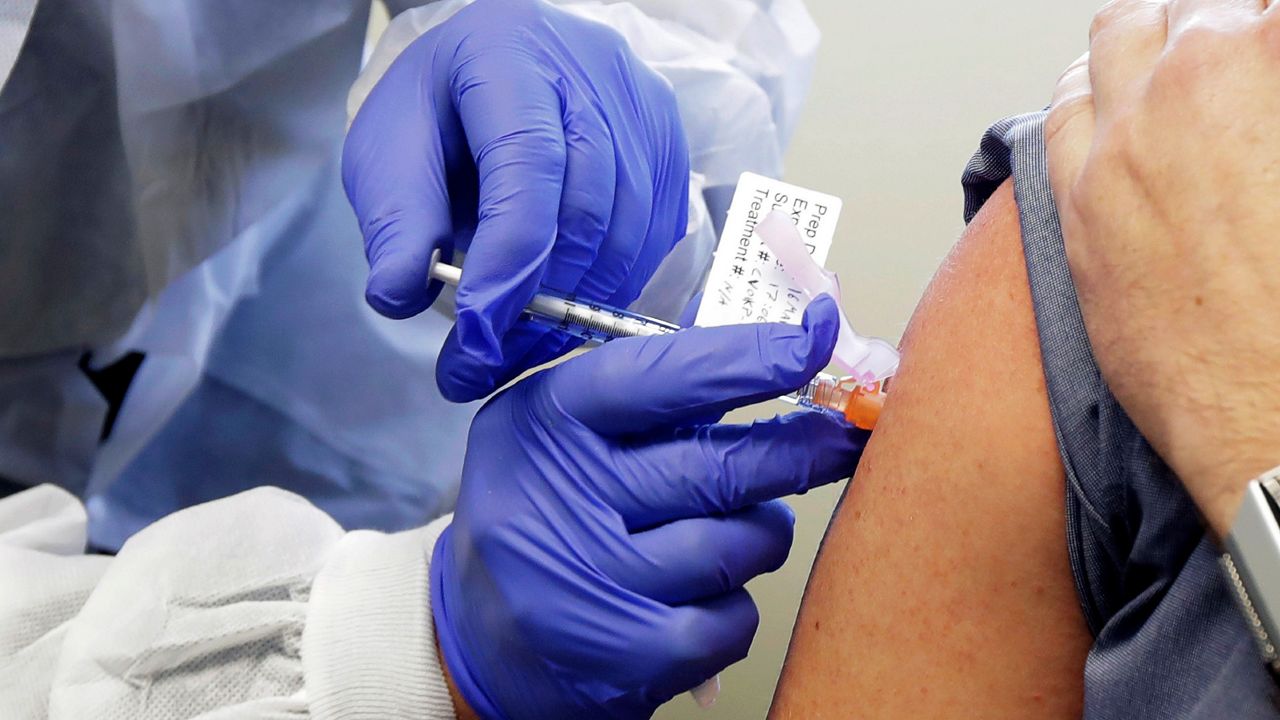
According to a release, six of Wake Research's locations have been selected to participate in Moderna’s phase 3 vaccine trial. The company is now one of the largest Phase I-IV clinical trial service companies in North America.
“As we continue our company’s participation in these trials offering ground-breaking investigational vaccines or treatment to COVID patients (inpatient and outpatient) across the country, our entire team is working diligently to assure patient safety in compliance with CDC and local public health authorities’ guidelines, while maintaining the highest quality and integrity metrics, and streamlining processes to ensure the most efficient and effective patient care and recruitment rates. Our best defense against the global pandemic is efficient and well-run clinical trials providing high integrity data toward discovery of new treatments,” said Ella Grach, MD, President and Chief Executive Officer of Wake Research.
Volunteers can inquire and start the enrollment process simply by texting “COVID” to the appropriate number for their respective location.
Locations for Wake Research COVID-19 clinical trials in North Carolina include:
Raleigh
COVID-19 Outpatient Vaccine, Treatment, and Testing. Wake Research site – 3100 Duraleigh Rd., Suite 304, Raleigh, NC 27612. For more information, text COVID to 919-276-8331.
Fayetteville
COVID-19 Outpatient and inpatient Vaccine, Treatment, and Testing. Carolina Institute for Clinical Research - 1841 Quiet Cove, Suite 2, Fayetteville, NC 28304. For more information, text COVID to 910-463-5578.
Other sites across the country include:
Chattanooga, TN
COVID-19 Outpatient Vaccine, Treatment, and Testing. ClinSearch. 6035 Shallowford Rd., Suite 109, Chattanooga, TN 37421. For more information, text COVID to 423-418-8464.
San Diego, CA
COVID-19 Outpatient Vaccine, Treatment, and Testing. Medical Center for Clinical Research. 9040 Friars Rd., Suite 540, San Diego, CA 92108. For more information, text COVID to 619-330-1172.
Dallas, TX
COVID-19 Outpatient Vaccine, Treatment, and Testing. Global Medical Research. 2701 S. Hampton Rd. Suite 250, Dallas TX, 75224. For more information, text COVID to 972-573-9490.
Las Vegas, NV
COVID-19 Outpatient Vaccine, Treatment, and Testing. Clinical Research Center of Nevada. 1012 E Sahara Ave, Las Vegas, NV 89104. For more information, text COVID to 702-357-9905
Volunteers interested in learning more about enrolling in a study can click here. If selected to participate in a study, patients are compensated for their time and travel.
“In addition to receiving the highest quality of care from some of the best physicians in the clinical research field, participants in these studies are contributing to the future of medicine, providing key insights and data needed to develop a treatment or vaccine for COVID-19,” Grach said.