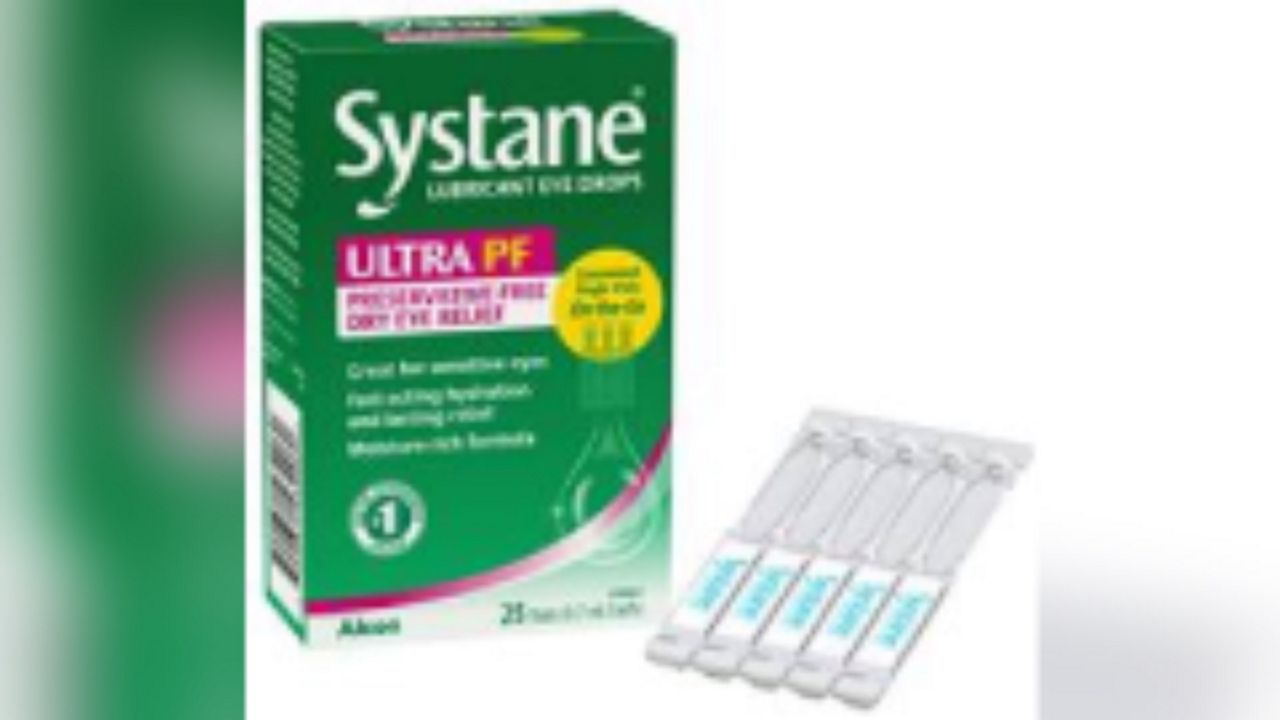
The Hawaii Department of Health Food and Drug Branch issued an alert Thursday on behalf of Alcon Laboratories for its Systane Lubricant Eye Drops Ultra PF Single Vials On-the-Go (25 count) because of potential fungal contamination.
The eye drops are sold nationwide, including in Hawaii, and the recall is limited to lot number 10101. The FDB is working with local stores to ensure the recalled product is no longer sold.
Fungal contamination of an ophthalmic product might cause eye infections. If an infection occurs, it may threaten vision and, in very rare cases, may be life-threatening in people who are immunocompromised.
DOH urges anyone showing symptoms after using the recalled product to contact their health care provider.
As of Thursday, no one had reported adverse effects from the recalled product.
DOH advises people to check if they bought the recalled product and, if so, to stop using it.
The lot number and expiration date are printed on the bottom of the product packaging and are also stamped on each single-use vial. People who bought the recalled product may return it to the place of purchase for a replacement or refund.
Consumers with questions regarding this recall may contact Alcon Laboratories at 1-800-241-5999 (between 3:30 a.m. and 2 p.m. HST, Monday to Friday).
Product information:
- Systane Lubricant Eye Drops Ultra PF Single Vials On-the-Go 25 count
- UPC: 300651432060
- Lot: 10101
- Expiration: 2025/09