BELLEVILLE, Ill. – The St. Clair County Health Department (SCCHD) is investigating its first probable case of monkeypox and is waiting for confirmation from the CDC.
Health officials believe the probable case is associated with domestic travel. An investigation shows the individual has had minimal contact with the public, and any potential close contacts have been notified.
Illinois has reported 152 probably and confirmed cases of monkeypox since May. Many of those cases have been in the Chicago area. Just this week, St. Louis health officials announced its first probable case. There also were two cases in the Kansas City area and one recently reported in the Springfield area. Nationally, the CDC is reporting 1,053 cases as of July 13.
The SCCHD says it has not identified any additional cases in the county. Officials also say monkeypox does not spread as easily as the COVID-19 virus and at this time, there is no indication that there is a great risk of extensive local spread of the virus.
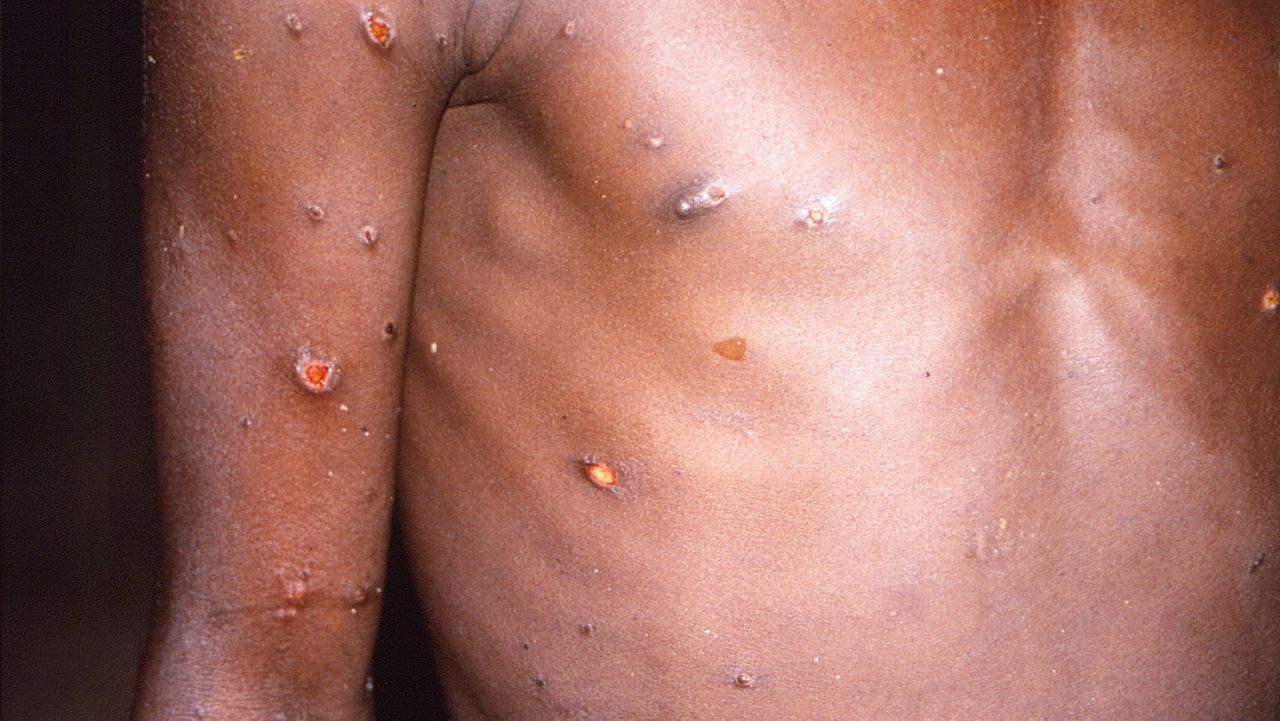
Person-to-person transmission is possible through close physical contact with body fluids, monkeypox sores, or items that have been contaminated with fluid or sores.
Monkeypox symptoms include fever and headaches, muscle aches and swollen lymph nodes, rash, bumps, or blisters that can appear anywhere on the body, including the genitals. This may look similar to syphilis, herpes, or other common skin rashes.
As the number of cases continue to rise across the country, thousands more doses of monkeypox vaccine are expected to soon begin shipping to the U.S. after federal health officials said they had completed an inspection of the overseas plant where they were manufactured.
The update from the Food and Drug Administration comes amid soaring demand for the two-dose vaccine as thousands of people in New York City, California and other parts of the U.S. await a chance to get the shot.
More than 1.1 million doses of the vaccine purchased by the U.S. government are currently at Bavarian Nordic's facility in Denmark. The company said earlier this week it needed authorization from an on-site FDA inspection before it could ship them to the U.S.
An FDA spokeswoman said late Wednesday that regulators “expedited and completed an inspection of the company's plant.”
“We do not expect any delay in vaccine availability due to this process,” she said in an emailed statement.
Bavarian Nordic has already shipped 300,000 vaccine doses that were made at a third-party facility that had previously been authorized by the FDA.
The Associated Press contributed to this story.