NATIONWIDE – The FDA has announced a voluntary recall of two brands of Infants’ Ibuprofen.
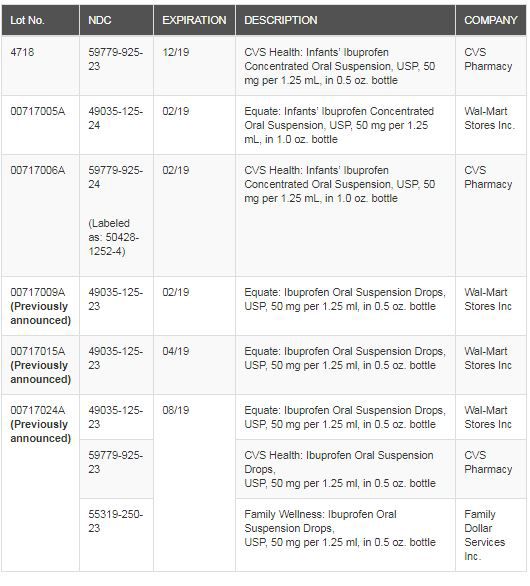
The recall includes Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 mL, and has been issued because some units from these batches have been found to have higher levels of Ibuprofen concentration.
The recall affects CVS and Equate brands and a specific list can be found below.
FDA
So far no serious adverse events have been reported related to this recall.
